The research team led by Professor Liu Qinghuai from Jiangsu Provincial People's Hospital and Professor Gu Yonghui from Suzhou Municipal Hospital recently published a study titled "Adaptive-optics scanning laser ophthalmoscopy study of non-arteritic anterior ischemic optic neuropathy" in Photodiagnosis and Photodynamic Therapy, utilizing MONA Adaptive-Optics Scanning Laser Ophthalmoscopy (AO-SLO) technology.
Introduction
Non-arteritic anterior ischemic optic neuropathy (NAION) is the most common acute optic neuropathy in adults over 50. Its pathogenesis remains unclear but is linked to optic disc hypoperfusion, causing irreversible vision loss, emphasizing the need for early diagnosis.
NAION damages retinal ganglion cells (RGCs), thinning the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL). OCT and OCTA quantify these changes, aiding in disease assessment. However, conventional imaging lacks the resolution to clarify NAION’s impact on photoreceptors.
The MONA adaptive optics system (Robotrak Technologies) achieves cellular-level resolution, enabling in vivo retinal cell imaging. It has been used in diabetic retinopathy, glaucoma, and inherited retinal diseases, but its application in NAION remains underexplored.
Methods
The observation group included 50 eyes from 27 NAION patients. After excluding 15 eyes with poor image quality, 35 eyes were included in the statistical analysis. Among these, 26 eyes were from 13 patients with unilateral NAION, further categorized into acute NAION (disease duration <2 months) and chronic NAION (disease duration >2 months) groups. The control group consisted of 10 eyes from 10 healthy individuals, matched for age, laterality, and general health status.
Imaging Examination and Analysis:
OCT examination: Evaluated ganglion cell layer (GCL) thickness and peripapillary retinal nerve fiber layer (pRNFL) thickness.
AO-SLO examination: In each participant, a 2.4°×2.4° (~700×700 μm) field centered at 3° eccentricity from the fovea was imaged in the superior, inferior, nasal, and temporal quadrants to quantify cone photoreceptor density.
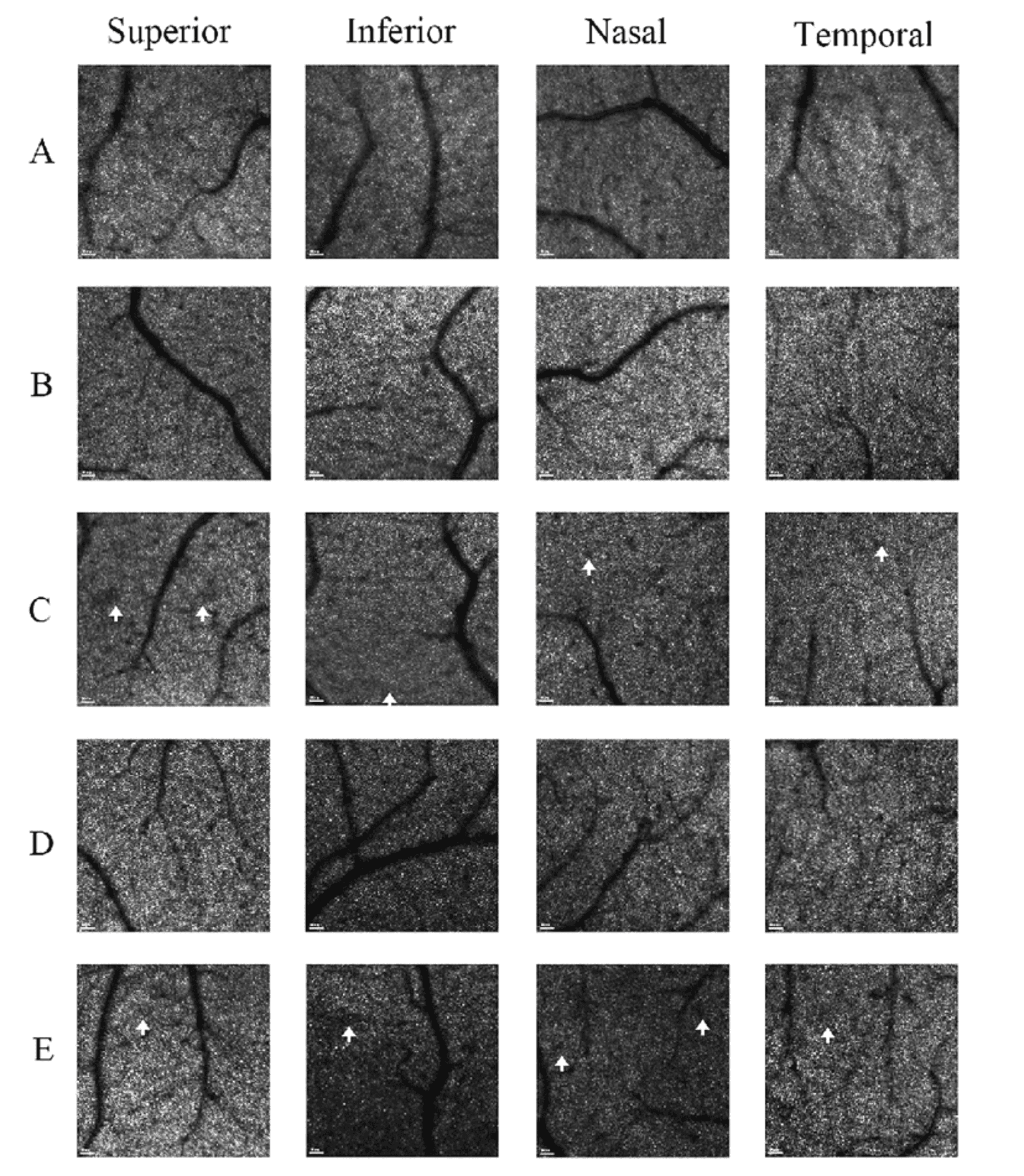
Result
Comparison of 2.4° × 2.4° AO-SLO cone cell density at 3° eccentricity of patients with monocular NAION
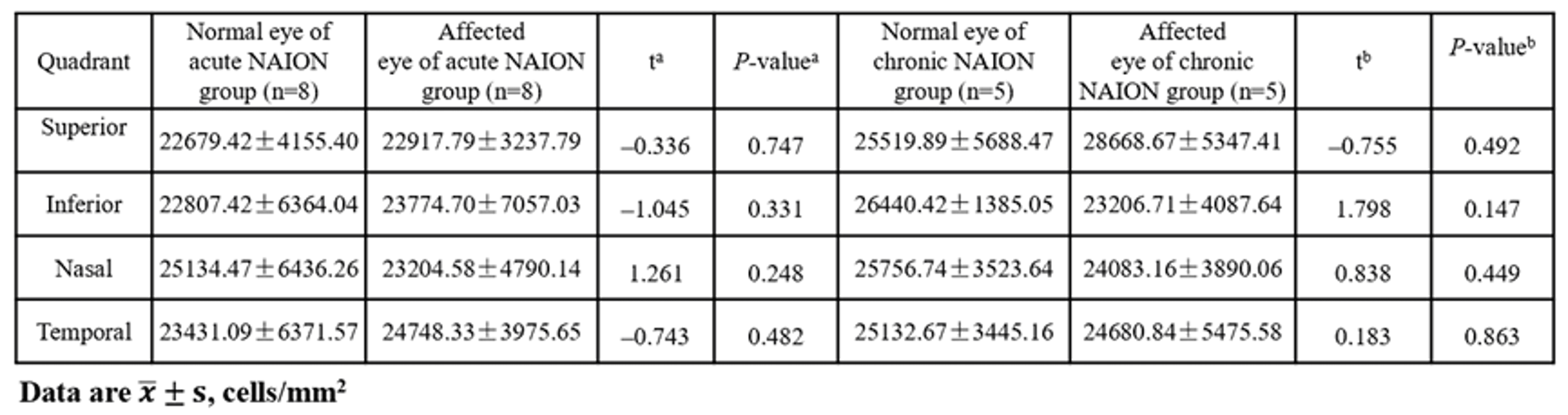
In both acute and chronic NAION groups, no significant differences (P > 0.05) were observed in cone photoreceptor density, regularity, or dispersion at 3° eccentricity across all four quadrants between the unaffected fellow eyes and the affected eyes.
Despite confirmed RGC and RNFL damage in affected eyes (as demonstrated by OCT), cone photoreceptor structure and distribution remained unaffected, suggesting that ischemic injury in NAION does not extend to the photoreceptor layer.
Comparison of 2.4° × 2.4° AO-SLO cone cell density at 3° eccentricity of patients with NAION and healthy participants.
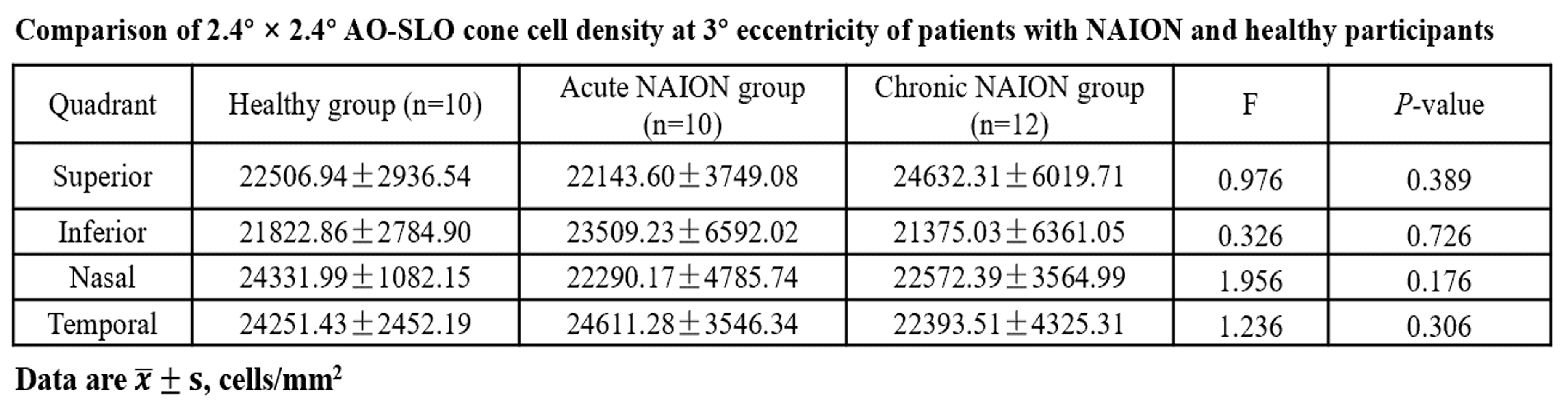
Among the healthy control group, acute NAION group, and chronic NAION group, no significant differences were found in cone photoreceptor density at 3° eccentricity across all four quadrants (P>0.05);
Acute NAION patients showed no cone photoreceptor loss caused by short-term ischemia;
Even after long-term optic nerve damage in chronic NAION patients, cone photoreceptors remained stable, excluding the impact of "trans-synaptic degeneration" on photoreceptor cells.
Discussion
AO-SLO imaging confirms preserved cone photoreceptor structure in NAION patients, while OCT demonstrates RGC and RNFL damage. This complementary evidence reveals NAION's selective pathology: optic nerve pathway impairment with intact photoreceptors, explaining the clinical observation of vision loss despite normal photoreceptor morphology.
The structural preservation of cone photoreceptors indicates maintained light detection capability. However, visual dysfunction occurs due to compromised signal transmission through damaged RGCs and RNFL. These findings establish RGC protection as the primary therapeutic target, suggesting that early intervention to prevent RGC loss may preserve functional visual pathways.
The MONA AO imaging system provides microscopic insights into NAION pathogenesis. Future integration with AI and machine learning could enhance clinical applications, enabling improved patient screening, progression prediction in unilateral cases, and optimized diagnostic workflows.
Article Link: https://doi.org/10.1016/j.pdpdt.2025.104678
